Abstract
Pichia guilliermondii strain Z1 and Bacillus subtilis strain ZH2 were previously selected for their high and reliable antagonistic activities against Penicillium digitatum (Sacc.) and blue mould (P. italicum Weh.) on citrus fruits. The effectiveness of pilot testing of wettable and a granular powder formulation based on each biocontrol agent was evaluated and compared to thiabendazole in two packinghouses. Each fruit was wounded on the outer surface on two opposite locations. Fruits intended to be treated with biofungicides were recovered just before applying the wax at packaging line. Fruits were soaked in a solution made with each formulation. Pathogenic inoculation was made 24 hours later by spraying two different varieties of fruits, Clementine ‘Nules’ and ‘Valencia-late’ orange, with a suspension of 105 conidia/ml of P. italicum or P. digitatum. The effect of treatment was evaluated after 7 days of fruit incubation at two temperatures 4 °C and 20 °C. The result showed that the control achieved with strain Z1 on ‘Valencia late’ orange was comparable to that with thiabendazole. On the other hand, the decay control was lower for all the treatments on the Clementine ‘Nules’. Nevertheless, ZH2 strain has no effect at 4 °C. Therefore, it was concluded that strain Z1 is a promising biocontrol agent for the control of major postharvest diseases of citrus in Moroccan packinghouse stations.
Keywords: Citrus, biological control, formulation, packinghouse, Pichia guilliermondii, Bacillus subtilis
INTRODUCTION
Citrus industry represents an important branch of the Moroccan agriculture. This sector is the main source of revenue for more than 10 000 growers and their families. It employs more than 32 millions labor days at various levels of production, transport and packing.
The total area planted with citrus in Morocco is estimated at 129,243 hectare (ha), of which there are 60.000 ha of oranges, 65,161 ha of small citrus fruits and 4626 ha of lime and others (Jaouad et al., 2020). The main producing regions are: Sous (Agadir), Molouya (Berkane), Haouz (Marrakech), Gharb (Kenitra), Tadla (Beni Mellal). Citrus production represents 2.6 million metric ton (Jaouad et al., 2020). More than 90 % of which are used either in the domestic or the export markets for fresh consumption. Fresh citrus exports totaled more than 715,450 tons. Main cultivated varieties are clementine-type varieties, such as ‘Nules’, ‘Cadoux’, ‘Nour’ and ‘Sidi Aissa’. Orange varieties are mainly dominated by ‘Valencia Late’ and Navels.
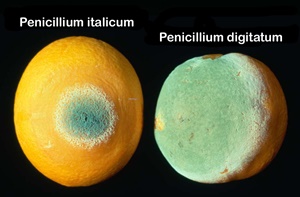
Postharvest losses of citrus fruits may reach very high values especially in the domestic market where fruits are not treated with fungicides and where storage techniques are not used most of the time. There are no studies which draw a clear idea of the extent of the disease losses after harvest. Some authors put the figure at 60 % loss (Taqarort et al., 2008; Lahlali et al., 2010). Blue and green mould caused by Penicillium italicum Wehmer and Penicillium digitatum Sacc., respectively, are the most common diseases that affect citrus fruit. Also, sour rot caused by Geotrichum citri-aurantii (Ferraris) E.E is more frequent in the degreenig rooms. Imazalil, thiabendazole and sodium o-phenylphenol (SOPP) are the most commonly used synthetic chemical compounds in the citrus packinghouse for the control of postharvest diseases during storage periods (Lahlali et al., 2014).
Nowadays, there is growing demand among consumers for fruits free of chemical residues harmful to human health. In addition, postharvest pathogens often develop resistance to fungicides. Therefore, there is a need for new and effective means of postharvest disease control that reduce risks to human health and the environment. Consequently, biological control of postharvest diseases of fruit has emerged as one of the most effective methods and may provide an effective eco-friendly alternative to synthetic chemicals (Droby and Chalutez, 1999; Wilson et al., 1991).
Several yeast and bacteria strains have been evaluated and reported as effective biocontrol agents of postharvest (Lahlali et al., 2005; Janisiewicz and Korsten, 2002; Palou et al., 2008; Lahlali et al., 2014). To date, there are some commercial products available on the market such as SHEMER WDG (Metschnikowia fructicola; Agro Green, Israel), Bio-Save 10 LP (Pseudomonas syringae Strain ESC-10; JET Harvest Solutions, Orlando, FL, USA), Candifruit (Candida sake, Valencia, Spain), and Boniprotect (Auerobasidium pullulans, Biofa AG, Germany) (Droby et al., 2009; Sharma et al., 2009).
The major step in the development and commercialization of biocontrol products is the development of an effective formulated product that retains biocontrol activity similar to that of the fresh cells. Therefore, the main objective of this study was to determine the efficacy of a wettable powder formulation of ZH2 and a granular formulation of Pichia guilliermondii strain Z1 to control blue and green mold respectively caused by P. italicumnd and P. digitatum in two Moroccan pakinghouses.
MATERIAL AND METHODS
Preparation of the Biocontrol agents
Pichia guilliermondii strain Z1 and Bacillus subtilis strain ZH2 were isolated from healthy Citrus fruits and identified by Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) (Germany).
The biomass production of strain ZH2 of Bacillus subtilis was carried out at 30 °C in a 200 litres Biostat ® 300 DS bioreactor (B. Braun Biotech, Germany). The medium contained per liter: 100 ml sporulation stock solution (0.6 g L-1 MnSO4 H2O, 0.5 g L-1 CuSO4 5H2O, 0.9 g L-1 ZnSO4 7 H2O, 0.09 g L-1 FeSO4 7 H2O), 20 g L-1 Nutrient Broth, 10 g L-1 yeast extract, 5 g L-1 Tryptone, 4 g L-1 casein peptone, 0.2 g L-1 K2HPO4, 1 g L-1 MgSO4 7H2O and 5 g L-1 NaCl. 440 g glucose and 44 g CaCl2 2H2O sterilized separately and added aseptically. The medium was continuously aerated with 0.5 vvm, the stirring speed was maintained at 400 rpm. The pH of culture was controlled at 7. This strain is formulated under powder form (9.8 × 1010 spores g-1) by spray-drying (Figure 1A) (Mounir, 2007).
Biomass production of strain Z1 (Figure 1B) was conducted at 28 °C in a 10 l Biostat ED Bioreactor (B. Braun Biotech. Germany). The culture medium used contains 50% glucose (w/w) as a carbon source, amino acids (30 g yeast extract and 30 g soy peptone), and either 5 ml of mineral salts concentrated medium or 5 ml of sterilized concentrated vitamin solution for growth. The biomass production in relation to the batch system was increased using feed-batch technology (Biotechnology Unit, Laboratory of Microbiology, ULB, Belgium). The produced biomass was dried in a fluid bed dryer. Maize starch was used as a loading agent (30%). Air temperature in the bed was maintained at 30°C with air inflow at 150 m3/h throughout the drying process (Mounir, 2007).
Figure 1:Formulated products of the antagonists Bacillus subtilis ZH 2 (A) and Pichia guilliermondii Z1 (B) formulated products
Pathogen Preparation
P. italicum, and P. digitatum were originally isolated from decayed citrus fruits. For long term storage, the pathogen was maintained in 25% glycerol at -80 °C. Before the experiment, it was recovered from the glycerol and grown on potato dextrose agar (PDA) medium (Merck, Darmsdt, Germany). The conidial suspensions were prepared from a ±7 days-old pathogen culture by recovering the surface of the colonies in 20 ml of 0.05% Tween 20. The content was filtered, homogenized and serially diluted in SDW to determine the cell count using Bürker’s cell.
Packinghouse effectiveness tests of biofungicde formulations
Pakinghouse tests were conducted in two different packinghouses sites: Delassus packinghouse located in Casablanca and APLAG packinghouse in Sidi Kacem (Gharb region). The standard layout of the packinghouse processing line is shown in figure 2. At the packinghouse station, the fruit received an extansive washing with tap water followed by automatic or manual pouring, pre-sorting and elimination of small fruit, pre-drying, and brushing. Fruits undergo two treatment fungicides one at a Drencher and another during the application of the wax (fungicide is mixed with the wax). The layout of the packaginghouse processing line is the same at both stations with the exception of absence of drencher at APLAG packinghouse station. Formulations of both biofungicdes of strain Z1 and ZH2 were evaluated separately. The objective of the packinghouse tests is to offer an alternative to the fungicide treatment applied with the wax. For each test, fungicide free fruits were collected from the packing line just before the wax application. As the inoculation of citrus fruit was not possible at packinghouse stations due to risk of contamination, in all cases the fruit were brought to the laboratoray and that is where inoculation procedure was performed following the standard protocol. Fruit, from Delassus packinghouse, were collected just before the fungicide and wax treatment. These fruits were already treated with fungicide at drencher and then washed, while those from APLAG packinghouse station did not received any fungicide treatment.
Fruit were wounded at two equidistant points at the equatorial site. Each wound was 5 mm in diameter and 4 mm in depth. Fruits were divided into lots: water treatments (Control), strain Z1, strain ZH2 and PT normal commercial treatment in packinghouse. The inoculation of fruit by the pathogenic fungus P. italicum or P. digitatum was done by spraying the spore’s suspension on the fruit. Fruits were kept at 20 ± 1 °C or 4 °C ± 1 °C for 7 days before disease evaluation. For both stations, the packinghouse fungicide treatment is consisted of a mixture of Imazalil (1000 ppm) and thiabendazole (1000 ppm) with wax. These packinghouse tests were perfomed on two citrus varieties ‘Valencia-late’ orange and clementine ‘Nules’. All these steps are summarized in figure 3.
Figure 2:Schematic diagram of the packinghouse line steps at DEALASSUS and APLAG packinghouse stations in Morocco
Figure 3:Layout of different important steps applied for the evaluation of biofungicide formulations for effective control of major postharvest diseases of citrus fruit on parckinghouse stations in Morocco
Statistical analysis
All trials were arranged in a randomized complete block design with three replicate per treatment and each trial ws repeated twice. The datasets were analyzed by the analysis of variance (ANOVA) procedure of the statistical analysis system (SAS Institute, version 9.1, Cary, NC, USA) and the means were compared using Fisher’s LSD test at statistical significance of P = 0.05.
RESULTS
All the treatments significantly reduced the percentage of wounds infected compared to the untreated control (Figures 4A and 4B). The infection rate on the treatment at 20 ±1°C was 90 %. The wettable powder formulation of Bacillus subtilis strain ZH2 significantly reduced the incidence of decay on ‘Valencia late’ fruits. The granular formulation of Pichia guilliermondii strain Z1 performed as well as the packinghouse treatment: 1000 ppm Imazalil with 1000 ppm Thiabendazole incorporated in wax (Figure 4A).
At 4°C the wettable powder formulation of the strain ZH2 was not effective however the strain Z1 formulation gave a better control but less than that of the packinghouse treatment (Figure 4B). In the case of APLAG packinghouse test on ‘Valencia-late’, both treatments significantly reduced the percentage of wounds infected compared to untreated control (Figures 5A and 5B). Pichia guilliermondii strain Z1 was less effective than the packinghouse treatment, 1000 ppm Imazalil and 1000 ppm Thiabendazole incorporated with wax, either against Penicillium digitatum or P. italicum. However, Delassus Packinghouse Test on ‘Nules’ fruits on ‘Nules’ variety, the percentage of infected wounds for both pathogens was reduced significantly with all the treatment (Figures 6A and 6B). The granular formulation of the Z1 strain performed less than the Imazalil treatment. The P. digitatum caused more infected wounds than P. italicum in all the treatments.
Figure 4:Effect of the treatments on the incidence of decay on wounded ‘Valencia late’ oranges artificially inoculated with Penicillium digitatum (A) and P. italicum (B) and incubated at 20 ± 1°C for 7 days at DEALASSUS packinghouse. PT: Packinghouse treatment (1000 ppm Imazalil +1000 ppm Thiabendazole with wax, Z1 formulated product: 108 CFU/ml. ZH2: formulated product 109 CFU/ml. Columns with the different letter are significantly different at p = 0.05 according to LSD test.
Figure 5:Effect of the treatments on the incidence of decay on wounded ‘Valencia late’ oranges artificially inoculated with Penicillium digitatum (A) and P. italicum (B), and incubated at 20 ± 1 °C for 7 days at APLAG packinghouse. PT: Packinghouse treatment (1000 ppm Imazalil +1000 ppm Thiabendazole with wax, Z1 formulated product: 108 CFU/ml. Columns with the different letter are significantly different at p = 0.05 according to LSD test.
DISCUSSION
B. subtilis strain ZH2 formulated product significantly reduced blue mold on ‘Valencia-late’ oranges when incubation was done at 20 ± 1 °C. However at 4°C this product showed no protective activity. Low temperatures are often used for citrus fruits preservation. The antifungal mechanisms involved in biological control ability of ZH2 against P. italicum of citrus fruits are mainly based on antibiosis (Drider et al., 2006). Consequently, it is necessary to optimize in the formulation process of both metabolites and cell production. Previous studies conducted on Bacillus subtilis strain CPA-8, demonstrated that treatments with culture (cell + endospore + antifungal metabolites in broth growth medium), cells and cell free supernatants from CPA-8 are effective to control brown rot on stone fruits (Yánez-Mendizábal et al., 2011). Although all these treatments provided an effective decay control, cultures showed better disease reduction than cell suspensions or cell free supernatants due to the combined action of vegetative cells, endospores and antifungal metabolites present in the medium. Pryor et al. (2007) also indicated that in vitro production of antifungal metabolites could have an immediate effect on the pathogen population post application particularly lipopeptides during B. subtilis fermentation. In other hand, in vitro production of bacterial cells (cells and endospores) could allow for potential in situ lipopeptide production; while the combination of both in vitro and in situ lipopeptide production could allow a maximum of efficacy. This may suggest that one of these components involved in the protective ability of this specie may be affected by low temperature.
This work highlights a significant suppression of blue and green molds by P. guilliermondii strain Z1 formulated product on ‘Valencia-late’ and ‘Nules’ fruits. This yeast species has been widely reported as an effective BCA against fungal pathogens, including blue and green mould of citrus fruits (Droby et al., 1993; Kinay and Yildiz, 2008), tomato spoilages (Saligkarias et al., 2002; Zhao et al., 2010) and Colletotrichum capsici on chilli fruit (Nantawanit et al., 2010). These results reinforce the ability of strain Z1 to survive and multiply in wounded sites. Pichia guilliermondii strain Z1 cell survival in wounded citrus fruits demonstrated a good adaptation of this BCA to cold storage temperatures. The yeast cell number at 5 °C was inferior to that observed at 25 °C (Lahlali et al., 2010). Furthermore, strain Z1 was able to grow at the wounded sites which are the main entry points of postharvest citrus diseases. However, on ‘Nules’ variety the efficacy of this formulation was lower especially against the P. digitatum specie. On this citrus variety, even chemical treatment carried out by the packinghouse did not show a complete control of decay. The inoculation tests used in this study were very severe.
Figure 6:Effect of the treatments on the incidence of decay on wounded Clementine ‘Nules’ fruit artificially inoculated with Penicillium digitatum (A) and P. italicum (B), and incubated at 20±1 °C for 7 days at DELASSUS packinghouse. PT: Packinghouse treatment (2000 ppm Imazalil with wax, Z1 formulated product: 108 CFU/ml. Columns with the different letter are significantly different at p = 0.05 according to LSD test.
CONCLUSION
In summary, our study demonstrates that effective biocontrol against the main postharvest disease of citrus fruit could be achieved by strain Z1 granular formulation. This strain Z1 had been found to be compatible with many waxes used in citrus packinghouse processing line (Lahlali et al., 2014). However, the possibility of applying this antagonist in combination with commonly used waxes in citrus packinghouse stations need further investigation.
REFERENCES
Abadias M., Benabarre A., Teixidó N., Usall J., Viñas I. (2001). Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. International Journal of Food Microbiology, 65:173-182
Abadias M., Usall J., Teixidó N., Viñas I. (2003). Liquid formulation of the postharvest biocontrol agent Candida sake CPA-1 in isotonic solutions. Phytopathology, 93:436-442.
Drider R., Friel D., El Guilli M., Jijakli M.H., Ibriz M. (2006). Study on the mode of action of two biocontrol agents Z1 and Zh2. IXth meeting of the phytopathogens group: Fundamental and practical approaches to increase biocontrol efficacy. September 6-10 Spa-Belgium.
Droby S., Chalutez E. (1999). Biological control of postharvest decay in citrus fruit. pp. 107–122. In: Marioschira (Ed.), Advances in postharvest diseases and disorders control of citrus fruit. Research Signpost, Trivandrum, India. European Journal of Plant Pathology, 123:37–45.
Droby S., Hofstein R., Wilson C.L., Wisniewski M., Fridlender B., Cohen L., Weiss B., Daus A., Timar D., Chalutz E. (1993). Pilot testing of Pichia guilliermondii: a biocontrol agent of postharvest diseases of citrus fruit. Biological Control, 3:47–52.
Jaouad M., Moinina A., Ezrari S., Lahlali, R. (2020). Key pests and diseases of citrus trees with emphasis on root rot diseases: An overview. Mor. J. Agri. Sci., 1(3): 149-160
Kinay P.,Yildiz M. (2008). The shelf life and effectiveness of granular formulations of granular formulations of Metschnikowia pulcherrima and Pichia guilliermondii yeast isolates that control postharvest decay of citrus fruit. Biological Control, 45 :433–440.
Lahlali R., Hamadi Y., El Guilli M., Jijakli MH. (2011). Efficacy assessment of Pichia guilliermondii strain Z1, a new biocontrol agent, against citrus blue mould in Morocco under the influence of temperature and relative humidity. Biological Control, 56:217–224.
Lahlali R., Serrhini M.N., Jijakli M.H. (2005). Development of a biological control method against postharvest diseases of citrus fruits. Communications in Agricultural and Applied Biological Sciences, Ghent University, 70 :47–58.
Lahlali R., Hamadi Y., Drider R., Misson C., El Guilli M., Jijakli M.H. (2014). Control of citrus blue mold by the antagonist yeast Pichia guilliermondii Z1: Compatibility with commercial fruit waxes and putative mechanisms of action. Food Control, 45: 8-15.
Nantawanit N., Chanchaichaovivat A., Panijpan B., Ruenwongsa P. (2010.) Induction of defense response against Colletotrichum capsici in chili fruit by the yeast Pichia guilliermondii strain R13. Biological Control, 52: 145–152.
Mounir R. (2007). Etude de la sélection, la production en masse et la formulation d’agents de lutte biologique contre les pathogènes de post-récolte des pommes et des agrumes. 132 p.
Obagwu J., Korsten L. (2003). Integrated control of citrus green and blue moulds using Bacillus subtilis in combination with sodium bicarbonate or hot water. Postharvest Biology and Technology, 28:187–194.
Palou L., Usall J., Munoz A., Smilanick J.L., Vinas I. (2002). Hot water, sodium carbonate, and sodium bicarbonate for the control of postharvest green and blue molds of Clementine mandarins. Postharvest biology and Technology, 24: 93–96.
Pryor S.W., Gibson D.M., Hay A.G., Gossett J.M., Walker LP. (2007). Optimization of spore and antifungal lipopeptide production during solid-state fermentation of Bacillus subtilis. Applied Microbiology and Biotechnology, 143: 63–79.
Saligkarias D., Gravanis F.T., Epton H.A.S. (2002). Biological control of Botrytis cinerea on tomato plants by the use of epiphytic yeasts Candida guilliermondii strains 101 and US 7 and Candida oleophila strain I-182: I. in vivo studies I. Biological Control, 25: 143–150.
Sharma R.R., Singh R., Singh R. (2009). Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biological Control, 50: 205–221.
Taqarort N., Echairi A., Chaussod R. et al. (2008). Screening and identification of epiphytic yeasts with potential for biological control of green mold of citrus fruits. World Journal of Microbiology and Biotechnology, 24:3031–3038.
Vero S., Mondino P., Burgaeno J., Soubes M., Wisniewski M. (2002). Characterization of biological activity of two yeast strains from Uruguay against blue mold of apple. Postharvest Biology and Technology, 26: 91–98.
Yánez-Mendizábal V., Usall J., Viñas I., Casals C., Marín S., Solsona C., Teixidó N. (2011). Potential of a new strain of Bacillus subtilis CPA-8 to control the major postharvest diseases of fruit. Biocontrol Science and Technology, 21: 409–426.
Yánez-Mendizábal V., Viñas I., Usall J., Torres R., Solsona C., Teixidó N. (2012). Production of the postharvest biocontrol agent Bacillus subtilis CPA-8 using low cost commercial products and by-products. Biological Control, 60 :280–289.
Zhao Y., Tu K., Tu S., Liu M., Su J., Hou,Y.P. (2010). A combination of heat treatment and Pichia guilliermondii prevents cherry tomato spoilage by fungi. International journal of Food Microbiology, 137:106–110.