Abstract
Corn is one of the three most important cereal crops in the world. The mites that attack maize belong to the family Tetranychidae, and the species Tetranychus urticae. Koch TSM and Oligonychus pratensis BGM (primary pests of cereals) are particularly formidable. Although high populations of spider mites frequently cause significant damage to corn (grain, silage, and sweet), the level of economic loss varies from season to season. Several factors influence population dynamics from year to year, including temperature, humidity, precipitation, soil type, pesticide applications, proximity to the host, and natural enemies. High temperatures and drought stress usually accelerate the accumulation process of high mite populations. This is compounded by the application of neonicotinoid pesticides, dust accumulation on corn leaves, and sandy soils as these soils are typically drought stressed, even with irrigation. Mites are responsible for severe yield losses of up to 40% of dry matter for silage. With the extensive and unsustainable use of pesticides, including acaricides and insecticides, management of mites currently commercially available miticides has become increasingly difficult, as these arthropods have developed resistance to over 95 active ingredients. The use of drought-tolerant corn plants can help reduce spider mite outbreaks and losses associated with these pests. Natural enemies, including predatory mites of the phytoseiidae family (Phytoseiulus persimilis Athias, Neoseiulus californicus), thrips (Scolothrips sexmaculatus), the tiny pirate bug (Orius sp), and the small black lady beetle (Stethorus punctillum) can keep spider mite numbers below the economic damage threshold. However, the effectiveness of these natural enemies is reduced by hot, low humidity conditions, pesticide use and dust accumulation on leaves. Proper irrigation can help reduce the risk of plant drought and the environment in which spider mites thrive. Eliminating alternate hosts for grasses can reduce their population potential. These biological and cultural control practices can be beneficial but often unreliable, which is why spider mite management on corn relies heavily on synthetic chemicals. New active ingredients such as etoxazole, dimethoate and fenpyroximate have been recentlyintroduced in the market to control spider mites more effectively. Preventive treatments at the beginning of the season can bring significant economic advantage. This effectiveness is greatly enhanced by aerial treatments. Electrostatic nozzles, for example, have been shown to be 3 times more effective than conventional hydraulic nozzles in controlling corn spider mites.
Keywords: Phytophagous mites, maize, natural enemies, synthetic chemicals, aerial treatment
INTRODUCTION
The increased frequency and intensity of abiotic and biotic stresses due to climate change will lead to a significant decline in agricultural production worldwide. Water stress is of particular concern, as it not only reduces crop yields by impairing plant growth and development, but also triggers outbreaks of herbivorous pests that thrive under such conditions. Spider mites, in particular, thrive in water-stressed maize crops and are responsible for severe yield losses (Gill, 2020). They not only feed on plant sap, but also spread plant pathogens and viruses, resulting in huge reductions in crop production (Yu et al., 2019). Commercial yield losses of up to 40% of maize dry matter destined for use as silage have been documented in Colorado, although normal losses are generally lower. Research on mites has shown grain losses ranging from 6 to 48%, with an average of 21% over 18 years (Peairs, 2012a).
The application of miticides is still one of the methods of prevention and control of agricultural mites, but the frequent use and misuse of miticides is causing more and more resistance in mites. To combat the growing problem of mite resistance, low-toxicity, effective and environmentally friendly miticides with unique structures and new mechanisms of action still need to be continuously introduced in the market (Yu et al., 2019). Environmentally safe alternatives and sustainable management of these pests in agricultural crops are needed, including those compatible with ecological or organic production systems. It is of utmost importance that new management tools are compatible with the use of natural enemies, both those of natural incidence and those implemented by farmers, minimizing negative effects on the environment and promoting biological balance (Miotto et al., 2020, Steiner et al., 2011). Due to increasingly frequent drought situations, the demand for water-stress tolerant crops is also increasing and agricultural companies are developing corn varieties with increased resistance to abiotic stressors. Drought-tolerant plant hybrids alleviate the challenges of growing crops in water-limited environments (Ruckert et al., 2021).
This review provides information on two-spotted spider mites, their population dynamics, scouting, treatment thresholds, and the choice of miticides for maize.
Main Mites on Corn
Numerous species of mites that specifically attach the ccorn crop have been identified. Two-spotted spider mites (Tetranychus urticae,TSM) and Bank grass mites (Oligonychus pratensis,BGM) are the two major pests of corn that have been associated with significant crop losses (Bui et al., 2018; Gunbharpur S. Gill, 2020).
T. urticae Koch (Tetranychidae) is one of the most polyphagous pests among herbivorous arthropods, feeding on more than 1150 plant species, belonging to 127 botanical families. Moreover, the grass mite (O. pratensis Banks) (Tetranychidae), perhaps the most damaging pest of maize grown in some semi-arid regions (Migeon & Dorkeld, 2019).
Morphology of TSM and BGM
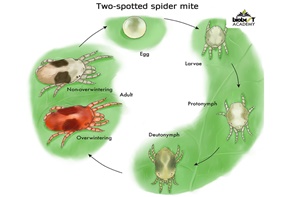
TSM and BGM are similar in shape and size (less than 1 mm), making it difficult to distinguish the two species with the naked eye. The TSM has a pale cream colour and two well-defined symmetrical black spots on the upper abdomen, while the BGM is pale green with blackish spots that extend to all sides of the abdomen. Males are generally smaller, slender and have a pointed abdomen, while females are more robust. Larvae have three pairs of legs, while proto- and deutonymphs, as well as adults, and have four pairs of legs (Ruckert, 2017).
Stages of Development
In spider mites, between the egg and the adult there are three active larval stages alternating with three resting stages. The egg gives rise to a hexapod larva, which feeds actively and then enters a first resting stage or protochrysalid. The next active stage is the protonymph, which is already octopod, followed by a second resting stage or deutochrysalid, and then the final larval stage or deutonymph, which differs from the protonymph in size. From the deutonymph stage onwards, the difference between the individuals that will give males and those that will give females begins to be established. It is finally the third stage of rest or teliochrysalid to which the adult follows.
The total duration of the developmental stages varies from about 6 days, under the most favourable conditions, to more than 1 month, depending on the species, temperature and hygrometry (Sabelis & Dicke, 1985). For Tetranychus urticae Koch, reared at a constant temperature of 25°C, at a constant hygrometry of 50%, the total duration of development is 9 days for males and 9.2 days for females. However, two-spotted spider mites can increase when temperatures are above 30°C and humidity levels are low. These are ideal conditions for two-spotted spider mites and populations are able to increase very rapidly (Gutierrez, 1991).
Longevity and Fertilization of Females
In rearing, at an average temperature of 25°C, female spider mites lay, depending on the species, 30 to 110 eggs over a period of 3 to 4 weeks, although oviposition and longevity can be increased by more than 50% when using techniques that are more difficult to implement, but more similar to natural conditions (Gutierrez, 1991).
Sex Ratio
The sex ratio in Tetranychidae varies from species to species, averaging 1 male to 3 females (Abdelgaleil et al., 2019). The sex ratio is genetically controlled, but the proportion of females decreases if the population density increases or the quality of the foliage decreases (Bolland et al., 1998).
Diapause
In temperate countries, most spider mites enter diapause during the winter season. This phenomenon occurs as a result of the simultaneous reduction in the period of sunlight and the lowering of the temperature from late summer onwards. Overwintering occurs as eggs in the genera Oligonychus and Panonychus and as overwintering females in the genera Eotetranychus and Tetranychus.
Winter females of Tetranychus urticae, which are yellow-orange in colour, have greater fat reserves than summer females and consume much less oxygen. They do not feed and do not lay eggs, finding shelter in crevices, trunks or under bark; they can withstand a temperature of -27°C (Gutierrez, 1991).
Reproduction by Arrhenotopic Parthenogenesis
Reproduction by arrhenogenesis appears to be widespread in spider mites: males are born from an unfertilized haploid egg, females from a diploid egg. It occurs when there is a high risk of females remaining unfertilized, but also when populations are subject to significant pressure from predators, when adaptations are required with respect to both climatic factors and host plants. This process therefore ensures better survival and wider dissemination of populations, since a single egg is sufficient to start a strain and produce a female (Gutierrez, 1991).
Infestation
The mites overwinter on other grass hosts. They return to the maize either by walking short distances or by being carried by the wind on silk threads over longer distances. Infestations begin on the underside of the lower leaves and gradually move to the upper part of the plant. The grass mite is usually found in corn from the mid-whorl to the grain-filling growth stages, while the yellow mite is rare on corn before flowering.
Rapid growth of the mite population occurs most often after pollen release. Factors that favour grass mite infestations include host drought stress, high temperatures, low precipitation, low humidity, absence of yellow mite, absence of natural enemies, use of insecticides and adequate moisture for alternate hosts during the previous growing season. Most of these factors will also favour the accumulation of the yellow mite, although the optimum temperatures for the yellow mite (30 to 32°C) are lower than those for the grass mite (36 to 37°C) (Peais, 2012).
Scouting
A hand magnifier is often used to detect two-spotted spider mites. They can be mistaken for dirt spots by the naked eye. Mites can be collected by shaking the leaves on a piece of white paper, then looking for moving mites. Two-spotted spider mites often congregate at field edges, especially if there are weeds around the edges. If their presence is confirmed, then estimate the population throughout the field by following a “Z” or “W” pattern (Hodgson, 2020).
Damage
Spider mites generally cause mechanical damage to plants and biochemical alterations, visible as chlorosis, leaf desiccation and abscission, growth inhibition and reduction in seed size (Schmidt, 2014). TSM and BGM cause chlorotic spots on corn leaves that can develop into necrotic areas, reduced grain moisture, grain shrinkage and, in some cases, stalk rot and plant lodging. However, the symptoms of a spider mite attack can vary and depend, among other things, on the species, the characteristics of the leaves and the specific reactions in the plant (Archer & Bynum, 1993). The saliva of spider mites can also trigger a toxic effect on the cells adjacent to the damaged cells, causing the destruction of their chloroplasts, degeneration of the nucleus and alterations in the structure of the cell walls. This damage reduces the synthetic capacity of the leaf and increases water loss. As their populations increase, the mites attack the upper leaves, abandoning the lower leaves, which they have dried out (Hodgson, 2020).
Plants infested with spider mites may have reduced levels of nitrogen, phosphorus and potassium, as well as altered levels of protein, carbohydrates and plant hormones, which can slow plant growth and reduce both productivity and product quality (Ruckert et al., 2021).
The Impact of Water Stress on Mite Populations
Climate change is expected to increase incidents of drought and produce more intense temperature patterns. Many studies have shown that high temperatures and dry conditions favour spider mite outbreaks. Under these conditions, the pest shortens its generation period and infestations build up rapidly as females produce more eggs that hatch quickly. TSM and BGM species, under optimal conditions of low humidity (20-40%) and high temperature (above 36°C), can increase their population size by about 70 per generation, and their generation period can be shortened by up to 10 days (Gill et al., 2020).. These are accompanied by morphological, physiological and biochemical changes in the plants, which may reduce their resistance to pests and favour the development of outbreaks of phytophagous arthropods (Chaves et al., 2003). It has been found that the tissues of stressed plants have a greater availability of nutrients, such as nitrogen and certain amino acids. Experiments carried out in this sense have shown that maize crops with optimal irrigation and those under water stress have different levels of infestation. Experiments carried out in the greenhouse and in the field have shown that water treatments (optimal irrigation and water stress) are followed by mite population growth (TSM and BGM) 7 days after the introduction of mites (Gill et al, 2020).
In the greenhouse, water-stressed plants experienced a 1.47-fold increase in mite population growth compared to optimally irrigated plants 7 days after mite introduction. Similarly, in the open field mite populations increased regardless of irrigation status for 7 days. It was 2.43 times higher on water-stressed plants than on optimally irrigated plants 7 days after mite introduction (Gill et al, 2020).
Water stress can also reduce protein biosynthesis, since growth is limited during periods of drought. Water-stressed plants experience reduced levels of defensive compounds against pests and pathogens. However, the production of defensive compounds may increase, depending on the intensity of the drought. The water treatment trials (optimal irrigation and water stress) also resulted in an increase in leaf temperature, leaf water potential and peroxydase (POD) activity in plants exposed to water stress. In addition, for optimally irrigated plants, CHI activity increased for TSM compared to BGM, and the relative increase in CHI and TI activities was much higher under water stress. However, the elevated CHI and TI activities were transient and returned to the levels observed for all other conditions (time points and mite species) by day 3. In contrast to the activities of POD, polyphenol oxidase (PPO), chitinase (CHI) and trypsin inhibitor (TI), which are widely conserved throughout the plant phylogeny, maize also produces specialized anti-herbivore compounds. Among these, benzoxazinoids such as 4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one (DIMBOA) and its derivatives are well known to deter insects, especially generalist species such as TSM. Higher TSM population growth on maize plants with mutations in the benzoxazinoid biosynthetic pathway. In contrast, BGM population growth was not affected, suggesting that BGMs have developed specialized defence mechanisms to overcome this major class of specialized compounds found in corn and several other major grass crops (Poaceae). In young plants, benzoxazinoid production is high in maize and declines rapidly with plant age, although benzoxazinoid production can be induced by herbivore damage to more mature plants in at least some maize lines.
Among the potential factors that may explain mite attacks on water-stressed maize, increased leaf temperature and altered leaf microclimate generally appear to enhance mite population growth by increasing fecundity and shortening the generation period.
In addition, drought promotes the dispersion of dust from adjacent roads onto crops, creating a more favourable habitat for spider mites by reducing the visibility of their natural enemies (Ruckert, 2017).
Mite Management in Corn
The economic damage that spider mites can cause varies from year to year and is dependent on several biotic and abiotic factors. The best way to manage spider mite infestations in a corn crop is to consider that biological, cultural and chemical control methods used individually or in combination will play a role in the decision (Pralavorio et al., 1989).
Host Plant Resistance in Maize for Spider Mite Management
Several studies screened maize inbred lines and identified several candidate plants showing spider mite resistance. In maize, antibiosis and tolerance resistance mechanisms have been reported against spider mites. Antixenosis is also a component of maize resistance as described (Singh & Seetharama, 2008). Among other cereals in the Poaceae family, benzoxazinoids are a class of plant defence compounds. Benzoxazinoids provide resistance against mites, insects, nematodes, fungi as well as bacteria. The resistant effects of benzoxazinoids are due to the anti-feeding properties driven by inhibition of proteases in the gut of herbivores. Of the many benzoxazinoid compounds, DIMBOA (2,4-dihydroxy-7-methoxy- 1,4-benzoxazin-3-1) is the primary compound showing these toxic effects and is stored in a non-toxic form; DIMBOA-glucoside, in the cell vacuoles (Meihls et al., 2012; Pereira et al., 2017). While benzoxazinoids are known to be present in young seedlings of plants, they can also be induced by herbivore feeding (Elek et al., 2013; Gianoli and Niemeyer, 1998; Maag et al., 2016). A two-year study of the effect of drought-tolerant corn hybrids exposed to the same irrigation treatments on artificially infested Banks’ spider mite on hybrids and resident mite populations showed that drought-tolerant, water-stressed hybrids had lower mite populations than drought-sensitive hybrids. Water-stressed drought-tolerant resident spider mite populations rant hybrids had Banks grass mite populations equal to drought-sensitive and drought-tolerant hybrids under optimal irrigation. This suggests that drought-tolerant hybrids can suppress spider mite outbreaks in water-stressed areas (Ruckert et al., 2021).
Monitoring
To prevent TSM and BGM infestations from reaching economically damaging levels, careful monitoring of corn plants throughout their growth cycle is recommended. Monitoring of these species begins after plant establishment and becomes more frequent over time, especially during the panicle formation and reproductive phase of the plant, when mite populations can develop rapidly (Chandler, 1979). It is recommended that leaf samples be taken from the lower third of the corn plant where spider mite infestations begin to develop. Leaves should be examined for yellowish-red brown spots, which are typical symptoms, webs and the presence of mites (Archer & Bynum, 1993, Ruckert, 2017). Currently, there is no predictive model to determine the timing of treatments for TSM and BGM in cornfields. Therefore, preventive control methods should be adopted, in addition to chemicals, to help mitigate the development of resistant spider mite populations (Ruckert, 2017).
Cultural Control
Irrigation
Proper irrigation is the key cultural practice in avoiding mite outbreaks. , More frequent and consistent irrigation during dry conditions can significantly reduce the effect of drought stress on mite development. In addition, overhead irrigation systems (pivot and sprinkler) can mechanically eliminate spider mites from the canopy or delay their development. However, once mite infestations are established, irrigation cannot reduce mite densities in corn (Filgueira 2000, Ruckert et al. 2015).
Host plant management Climate
It has already been established that TSM and BGM overwinter on border vegetation or senescent crops. Therefore, weed management and rapid removal of host plants can help prevent spider mites from contaminating adjacent cornfields, which occur primarily by crawling or wind dispersal (Brandenburg & Kennedy, 1982).
Optimization of Nitrogen fertilizers
Reducing fertilizer application may also reduce the risk of spider mite outbreaks, as high concentrations of nitrogen have been found to enhance spider mite populations by increasing nutrient availability in plant tissues. Nitrogen fertilization tends to favour mite infestations, but reducing nitrogen application rates to control spider mites is not economically viable (Peairs, 2012b).
Biological Control
Intensive use of non-selective pesticides and high reproductive capacity of spider mites have greatly increased the development of resistance to this pest. This has led to an increase in the rate and number of pesticide applications to achieve satisfactory control of the mite. As a result, high levels of pesticide residues may be present in agricultural products, which may adversely affect human health. Therefore, many studies have evaluated the potential of plant-derived substances as safe alternatives to synthetic miticides for controlling plant-feeding mites. Essential plant oils and their constituents have been shown to control mites and other economically important agricultural pests (Abdelgaleil et al., 2019).
Natural Enemies
Preservation of biological control agents is critical to the successful management of mite problems in corn. Thirty-five species of natural enemies from 15 families of predatory insects, mites and spiders have been associated with mites on corn (Peairs, 2012a). A study conducted in Texas between 1981 and 1983 on predators of spider mites (Acari: Tetranychidae) in corn fields and adjacent vegetation found several species of predatory arthropods associated with spider mite infestations in corn (Table 1). The predators Orius insidiosus (Say), (Hemiptera: Anthocoridae) and Feltiella macgregori (Felt) (Diptera: Cecidomyiidae) were the most abundant predatory insects collected. The most common predatory arachnids associated with spider mites were Neoseiulus setulus (Fox) (Acari: Phytoseiidae) and Dictyna consulta Gertsch and Ivie (Araneida: Dictynidae) (Pickett, 2014). Several predators responded numerically to increased spider mite densities, usually after spider mites had reached damaging numbers. In addition to predatory insects, Neozygites floridana, a naturally occurring fungus, is a common pathogen that attacks spider mites and may be useful in controlling populations. Fungal growth on spider mites is favored when daily temperatures are below 30°C with high relative humidity (Pralavorio et al., 1989). Plants also emit volatiles from feeding herbivores, which may attract the natural enemies of the latter (Kessler & Baldwin, 2000; Kaplan, 2012; Kaplan & Lewis, 2010; Kaplan & Thaler, 2015; Gill, 2020).
Many fields are not treated each year because the mites are kept in check by a variety of predatory mites, ladybugs, tiny pirate bugs, lacewing larvae and thrips. The most important of these are the predatory mites of the Phytoseiidae family (Amblyseius fallacis, Neoseiulus persimilis Athias and Neoseiulus californicus), a tiny bug, O. insidiosus, and a small black ladybug called Stethorus (Peairs, 2012b). Hot, dry conditions in which biological control agents cannot keep up with the increasing mite population are a common cause of mite outbreaks. Some spiders in the families Argiopidae, Theridiidae, and Linyphiidae have also been found to prey on phytophagous mites. Under these conditions, the predation and reproductive rates of many mite predators decrease, making them unable to suppress mite populations. This is particularly a problem in drought-stressed corn (Ruckert, 2017).
Effects of Pesticides on Natural Enemies
Insecticide applications directed at other corn pests are another common cause of mite outbreaks. These kill beneficial insects and mites, which in many cases keep the pest mites under control (Gill et al., 2020). If an insecticide is required, monitor mite activity on the treated crop or consider including a miticide in the application (Peairs, 2012a; Ruckert, 2017).
Effects of Dust on Natural Enemy Activity
Under hot, dry conditions, the predation and reproduction rates of many mite predators decrease, making them unable to suppress mite populations. In addition, these conditions favour the accumulation of dust on leaves, which can create microhabitats that favour spider mites but not their natural enemies. In fact, under these conditions, the dust deposit on the leaves is higher, which reduces the visibility of natural enemies. This is accentuated by the dispersion of dust from adjacent roads onto the crop (Ruckert, 2017).
Chemical Treatment
Biological and cultural control practices can be beneficial but often unreliable, which is why mite management relies heavily on chemical controls. While chemical control can be an effective option, it is not without problems and concerns. It is often made difficult by the widespread resistance to miticides in TSM and BGM and the withdrawal of many active ingredients effective against mites. To combat the growing problem of insect resistance, low toxicity, effective and environmentally friendly miticides with unique structures and new mechanisms of action still need to be continually introduced to the market, which is a difficult task and a huge challenge for pesticide chemists (Yu al., 2019).
Treatment Thresholds
Exact treatment thresholds for spider mites in corn do not exist. Instead, the decision to treat should take into account the length of time the field has been infested, the density of mites, including eggs, the location of the mites on the plant, moisture conditions and the appearance of the plant (Hodgson, 2020).
Economic Thresholds
For spider mites, the economic threshold is reached when mite damage is visible in the lower third of the plants. Most often, mites can be found in the middle third of the plant. Economic threshold can also be reached when most plants have mites at or around the spike leaf and 15 to 20 percent leaf discoloration (Ruckert, 2017; Hodgson, 2020).
Time of Treatments
Treatment thresholds should be done when damage is visible in the lower third of the plant, or when colonies are present in the middle third of the plant and the corn has not yet reached the hard dough stage. Once the corn crop has reached the hard dough to pod stage, there will be no economic benefit from a miticide treatment (Peairs, 2012). Chemical applications for mites in corn are recommended after the VT stage (panicle emergence), especially if the field has a history of spider mite outbreaks, expected daily temperatures exceed 35°C, rainfall frequencies are limited, signs of drought stress are beginning to appear, mite predator densities are low, and feeding injury is evident in the first third of the plant (Peairs 2010; Ruckert et al. 2015).
Chemical control of spider mites is usually achieved with contact or systemic miticides. These products are generally more active on adults because, during moulting, juveniles remain inactive under the old protective skin and do not feed immediately. In this case, active ingredients that kill by ingestion are temporarily ineffective (Hodgson, 2020).
Preventive Treatments
Early season preventive treatments can provide some economic benefit. Growers should carefully consider: the amount of plants infested with small mite colonies, temperature and humidity, drought stress, predatory insect populations, and the history of mite infestations in the field. Again, proper pest identification is essential (Pralavorio et al., 1989).
Another more sophisticated guideline also considers the cost of treatment and the expected value of the crop based on the percentage of leaves infested and leaf area damaged. To use this table, the cost of control (miticide + application cost) and the expected crop value (bushel of grain/acre x market price) must be determined. Next, a two-step sampling method is used. First, an individual plant is selected, the green leaves are checked for the presence or absence of mites and the percentage of infested green leaves (first value in the table) is calculated. This shall be done ten times in different parts of the field. If the percentage of infested green leaves exceeds the control cost and the crop value, then the percentage of leaf area damaged should be determined. + application cost) and the expected crop value (bushel of grain/acre x market price) should be determined. Next, a two-step sampling method is used. First, an individual plant is selected, the green leaves are checked for the presence or absence of mites and the percentage of infested green leaves (first value in the table) is calculated. This shall be done ten times in different parts of the field. If the percentage of infested green leaves exceeds the cost of the check and the value of the crop, then the percentage of leaf area damaged should be determined (Pralavorio et al., 1989).
Miticides
The most common products used against spider mites in commercial maize production include the active ingredients spiromesifen, Propargite, hexythiozox, etoxazole, bifenthrin and zeta-cypermethrin, dimethoate and fenpyroximate) (Riedl et al., 2006; Irigaray & Zalom, 2007; Haviland et al., 2011).
• Spiromesifen is a contact product, with a translaminar movement, and affects all life stages, causing death by inhibiting lipid biosynthesis ((Nauen et al., 2003; Cloyd et al., 2009).
• Propargite is a contact miticide effective against adults and juveniles, and acts as an inhibitor of ATP synthesis (Martini et al., 2012; Ruckert, 2017).
• Both Hexythiozox and etoxazole are effective against eggs and juveniles and act as growth regulators. However, Hexythiozox is a contact miticide, while etoxazole is contact and translaminar (Cloyd et al., 2009; Martini et al., 2012).
• The mixture of bifenthrin and zeta-cypermethrin is used against adults and juveniles, and alters sodium channels when applied directly to mites (Ahmad et al., 2002).
• Dimethoate is a systemic and contact product, which kills both adults and juveniles by inhibiting cholinesterase functions (Ruckert, 2017).
• Fenpyroximate is a contact pesticide effective against all stages of spider mite development, and inhibits mitochondrial electron transport (Stumpf & Nauen 2001).
Effects of Chemical Treatments on Natural Enemies
Pesticide applications for spider mites can have adverse effects on natural enemy populations (Gill, 2020). Insecticide applications directed at other corn pests are another common cause of mite outbreaks. These kill beneficial insects and mites, which in many cases keep the pest mites under control. If an insecticide is required, monitor mite activity on the treated crop or consider including a miticide in the application. It is also important to use more selective insecticides that are less toxic to natural enemies and to reduce the number of applications to prevent the death of natural predator populations (Peairs, 2012a; Ruckert, 2017).
Relationship between Neonicotinoids and Spider Mites
Mode of Action of Neonicotinoids
Neonicotinoids are a class of systemic insecticides widely used in agriculture against a wide range of pests (Jeschke and Nauen, 2008). Neonicotinoids can be either translaminar; where the insecticide passes through the leaf tissue from one leaf surface to another, or systemic; where the insecticide is absorbed by the plant and transported to other plant tissues (Matsuda et al., 2001). Neonicotinoids are not phytotoxic and can be directly applied in the soil through irrigation systems, by foliar spray application, or as seed treatments (Elbert et al., 2008).
Effect of Neonicotinoids on Arthropods
Neonicotinoids have recently come under scrutiny because of several unintended consequences on certain arthropods, including bees and spider mites. Firstly, these insecticides have a relatively long persistence in soil and water, prolonging their uptake by wild plants and subsequent crop plantings. As a result, arthropods are exposed to sublethal concentrations of the insecticidal active ingredients (Van der Sluijs et al., 2013). Recent studies have shown that the nitrated neonicotinoids such as imidacloprid, clothianidin, thiamethoxam, nitempyram and dinotefuran can severely affect pollinators by disrupting their memory, foraging behaviour, nectar storage and by reducing queen survival (Scholer & Krischik, 2014). Neonicotinoids were the dominant class of insecticidal compounds used to treat maize seeds. Seed treated with clothianidin and thiamethoxam cause mortality of Harmonia axyridis larvae, non-target ladybirds, when feeding on maize (Ruckert et al., 2021).
Effects of Neonicotinoids on Spider Mites
Spider mite outbreaks have also been observed after neonicotinoid insecticide applications (Moser & Obrycki, 2009). They are generally associated with the unintended suppression of natural enemies following pesticide use. However, neonicotinoid insecticides were found to have little effect on spider mite predators. Specifically, no negative effects of imidacloprid were observed on predatory mites and lacewings. Therefore, it was hypothesized that neonicotinoids activate different plant-mediated mechanisms that lead to spider mite outbreaks. For example, female E. buxi mites laid nearly 40% more eggs when fed on treated elm foliage, but their fecundity did not increase when the mites were directly exposed to imidacloprid through skin applications and then fed on untreated foliage. This result suggests that the increase in reproductive performance was an indirect, plant-mediated effect of neonicotinoids. Another study showed that T. urticae directly exposed to imidacloprid spray formulations or fed on leaves of a systemically treated bean plant produced 10-26% more eggs in the first 12 days of their adult life and 19-23% more eggs as adults plus during adulthood compared to water treatment alone. The increase in egg production occurred immediately after exposure and lasted for approximately 15 days in treated mites. In mites exposed to imidacloprid by ingestion, the increase in egg production occurred only after 6 days and lasted until about day 18. Longevity was significantly higher in mites that had ingested imidacloprid compared to those that had been sprayed.
Active ingredients such as imidacloprid, clothianidin, and thiamethoxam altered the concentration of the phytohormones 12-oxo-phytodienoic acid, jasmonic acid (JA), and salicylic acid (SA). These neonicotinoids also suppressed the transcription of phenylalanine ammonia lyase (PAL), coenzyme A ligase (CoA ligase), trypsin protease inhibitor (TI), and chitinase, all of which are regulated by SA (PAL, CoA ligase, chitinase) and JA (TI). This physiological alteration was then followed by a significant increase in the spider mite population in both greenhouse and field experiments (Ruckert et al., 2021).
Aerial Application Methods with Electrostatic Nozzles against Spider Mites
The development of spray application technology for improved delivery of spider mite control products on maize is essential for successful control of spider mite pest populations. A major weakness of conventional spray application methods, which use gravitational and inertial forces to deposit pest control products on target plant surfaces, is environmental contamination from overspray and lack of spray coverage on the plant leaf surface, resulting in less effective control of pests living on the lower leaf surface (Law, 2001). Trials of treatments with electrostatic and CP11-TT (conventional) nozzles showed that after 14 days of treatment that NDVI values of treatments with the CP11-TT nozzle and the electrostatic nozzle were comparable and were higher than those without treatment. This indicates that the electrostatic spray at a rate of 9.3 L/ha was equivalent to that of the hydraulic nozzle with three times the rate (28.1 L/ha) for controlling spider mites in maize. Also the damage assessment revealed that the damage levels of the conventional hydraulic nozzle and the electrostatic nozzle were comparable to each other and were lower than the untreated control. It was also shown that the spray droplet spectrum was larger for the CP11-TT nozzle than for the electrostatic spray nozzle treatment, due to their higher spray rate (Martin & Latheef, 2019).
Several workers reported that spray droplet size significantly reduced spider mite mortality. Munthali (1984) found found that smaller droplets increased mortality of immature stages of T. urticae when 1% oil-based dicofol sprays were applied rather than larger droplets; however, regardless of droplet size, efficacy depended largely on better deposition of the active ingredient on the surface of target leaves. Further research is needed to evaluate the efficacy of the treatments reported here against these two spider mite species in feldspar studies, with emphasis on species identification, spray deposition and penetration, and droplet spectra (Martin & Latheef, 2019).
Resistance
Generality
Today, The development of insecticide resistance in insects is common and widespread . The long-term effectiveness of insecticides is critical to the success and sustainability of food and fiber production and public health. What’s more, the development of insecticide resistance eliminates management options and potentially reduces profitability for producers (Bilbo et al., 2020).
In cultivated areas, strong selection pressure is exerted by plant protection products and many cases of pesticide resistance have been identified in Tetranychidae and some of their predators Phytoseiidae. With the increase in acaricide resistant populations, spider mites have become increasingly difficult to manage with currently available commercial acaricides and resistance to over 95 active ingredients has been identified (Arthropod Pesticide Resistance Database (APRD), 2019). The level of resistance varies by species, location, and year. Although O. pratensis is generally considered less resistant to acaricides than T. urticae, it is highly resistant in areas with long periods of drought (Yu et al., 2019).
Many aspects of mite biology, including rapid development, high fecundity, and haplo-diploid sex determination, are factors conditioning the rapid evolution of resistance to commercial miticide (Miotto et al., 2020).
Hormoligosis
Hormoligosis episodes, in which sublethal amounts of pesticides induce reproductive stimulation in herbivorous arthropods, have been observed in mites, especially in spider mites. Residues of carbaryl and dioxacarb caused T. urticae to lay more eggs than when the species was maintained on untreated plants. T. urticae also showed increased egg production when directly exposed to imidacloprid spray formulations, or fed treated bean plants (James & Price, 2002). Other examples of hormoligosis were found on the red mite Panonychus citri McGregor after citrus plants were treated with malathion or permethrin (Ruckert, 2017).
Examples of Acaricides for which Spider Mites have Developed Resistance
Yang et al. (2008) observed the development of resistance in T. urticae and O. pratensis following the use of bifenthrin (pyrethroid) and dimethoate. This resistance is due to increased enzymatic hydrolysis and oxidation of the insecticide by the mites. T. urticae has recently shown resistance to bifenazate, an acaricidal active ingredient that belongs to the group of hydrazine derivatives and acts by altering the electron transport chain. T. urticae has also shown resistance to many other chemical families that inhibit mitochondrial electron transport such as quinazolines, pyrimidinamines, pyrazoles and pyridazinonesl. But also resistance to avermectins (Abamectin), which are obtained from the soil microorganism Streptomyces avermitilis Kim & Goodfellow (Bacteria: Streptomycetaceae) has been identified (Rückert, 2017).
Resistance Management
Due to the ability of spider mites to develop resistance to acaricides, resistance management is a major concern for growers. Continued use of a single miticide naturally selects for susceptible mites and increases the number of tolerant mites in each subsequent(Pralavorio et al., 1989).
Alternating chemicals with different modes of action, reducing the frequency of application, and adhering to product label rates are strongly encouraged. By following these practices, the development of spider mite resistance, as well as hormoligosis, can be prevented (Ruckert, 2017). Keeping corn well-watered can help avoid water stress. Planting Corn next to winter wheat and alfalfa fields should also be avoided, especially if mite infestations are known. Insecticides should only be used when there is a risk of significant yield loss. If acaricide applications are necessary, maximize acaricide activity by applying with the appropriate carrier and adjuvants. Finally, avoid using the same miticide year after year (Pralavorio et al., 1989).
Conclusion
The infestation of maize by spider mites is strongly linked to hot and dry conditions coupled with the frequent and unreasoned use of pesticides, especially miticides, which, on one hand, increase the phenomenon of mite resistance and, on the other, eliminate the natural enemies of these mites. Thus, appropriate irrigation can help to reduce the infestation of spider mites. Additionally, rational use of pesticides that takes into consideration the natural enemies of the mites can equally help with mites mitigation. Studies show that there are many products such as plant extracts and oils that can help control mites on maize and reduce the use of miticides. However, mite control is still necessary during heavy attacks and during certain periods when spider mites are present. All the products that have been tested are almost very effective. The efficacy of the products is most improved when they are sprayed by air using electrostatic nozzles. Managing spider mite resistance is a combination of different control methods.
References
Abdelgaleil, S. A. M., Badawy, M. E. I., Mahmoud, N. F., & Marei, A. E. S. M. (2019). Acaricidal activity, biochemical effects and molecular docking of some monoterpenes against two-spotted spider mite (Tetranychus urticae Koch). Pesticide Biochemistry and Physiology, 156: 105-115.
Ahmad, S., Veyrat, N., Gordon-Weeks, R., Zhang, Y., Martin, J., Smart, L., Glauser, G., Erb, M., Flors, V., Frey, M., (2011). Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol., 157: 317–327.
Ahmad M, Hollingworth RM, Wise JC (2002). Broad spectrum insecticide resistance in oblique banded leaf roller Choristoneura rosaceana (Lepidoptera: Tortricidae) from Michigan. Pest. Manag. Sci., 58: 834-838.
Archer, T. L., & Bynum, E. D. (1993). Yield loss to corn from feeding by the Banks grass mite and two-spotted spider mite (Acari: Tetranychidae). Experimental & Applied Acarology, 17: 895-903.
Bilbo, T. R., Reay-Jones, F. P. F., Greene, J. K., & Musser, F. (2020). Evaluation of Insecticide Thresholds in Late-Planted Bt and Non-Bt Corn for Management of Fall Armyworm (Lepidoptera: Noctuidae). Journal of Economic Entomology, 113: 814-823.
Bolland, H. R., Gutierrez, J., & Flechtmann, C. W. (1998). World Catalogue of the Spider Mite Family (Acari: Tetranychidae). August, 392.
Brandenburg RL, Kennedy GG (1982) Intercrop relationships and spider mite dispersal in a corn/peanut agro-ecosystem. Entomol. Exp. Appl., 32: 269-276.
Bui, H., Greenhalgh, R., Ruckert, A., Gill, G.S., Lee, S., Ramirez, R.A., Clark, R.M., (2018). Generalist and specialist mite herbivores induce similar defense responses in maize and barley but differ in susceptibility to benzoxazinoids. Front. Plant Sci., 1222.
Bynum Jr, E. D., Xu, W., & Archer, T. L. (2004). Potential efficacy of spider mite-resistant genes in maize testcrosses. Crop Protection, 23: 625-634.
Bynum Jr., E.D., Michels Jr., J., MacDonald, J.C., Bible, J.B., (2015). Impact of Banks grass mite damage to yield and quality of maize silage. Southwest. Entomol., 40: 251–262.
Chandler, L. D., Archer T. L., Ward C. R., and W. M. Lyle. (1979). Influences of irrigation practices on spider mite densities on field corn. Environ. Entomol., 8: 196- 201.
Chaves MM, Maroco JP, Pereira JS (2003). Understanding plant responses to drought from genes to the whole plant. Funct. Plant. Biol., 30: 239–264.
Cloyd, R. A., Galle, C. L., Keith, S. R., Kalscheur And, N. A., & Kemp, K. E. (2009). Effect of commercially available plant-derived essential oil products on arthropod pests. Journal of Economic Entomology, 102: 1567-1579.
Elbert A, Haas M, Springer B, Thielert W, Nauen R (2008). Mini-review - applied aspects of neonicotinoid uses in crop protection. Pest Manag. Sci., 64:1099-1105.
Elek, H., Smart, L., Martin, J., Ahmad, S., Gordon‐Weeks, R., Welham, S., & Werner, C. P. (2013). The potential of hydroxamic acids in tetraploid and hexaploid wheat varieties as resistance factors against the bird‐cherry oat aphid, Rhopalosiphum padi. Annals of applied biology, 162: 100-109.
Filgueira FAR (2000) Novo manual de olericultura: agrotecnologia moderna na produção e comercialização de hortaliças. Viçosa: UFV. 401p
Gianoli, E., Niemeyer, H.M., (1998). Allocation of herbivory-induced hydroxamic acids in the wild wheat Triticum uniaristatum. Chemoecology, 8: 19-23.
Gill, G. S. (2020). Interactions Between Water-Stress and Maize Resistance to Spider Mites with Varied Host Specialization (Doctoral dissertation, Utah State University).
Gill, G. S., Bui, H., Clark, R. M., & Ramirez, R. A. (2020). Varying responses to combined water-stress and herbivory in maize for spider mite species that differ in host specialization. Environmental and Experimental Botany, 177: 104131.
Glauser, G., Marti, G., Villard, N., Doyen, G. A., Wolfender, J. L., Turlings, T. C., & Erb, M. (2011). Induction and detoxification of maize 1, 4‐benzoxazin‐3‐ones by insect herbivores. The Plant Journal, 68: 901-911.
Gutierrez, J. (1991). Workshop on the cassava network in Cotonou: 4-8 March 1991. 4–8.
Hanhineva, K., Rogachev, I., Aura, A. M., Aharoni, A., Poutanen, K., & Mykkanen, H. (2011). Qualitative characterization of benzoxazinoid derivatives in whole grain rye and wheat by LC-MS metabolite profiling. Journal of agricultural and food chemistry, 59: 921-927.
Haviland D, Rill SM, Holtz BA (2011). Effects of insecticide treatments for navel orange worm on populations of Pacific spider mite in almond, 2010. Arthropod Management Tests, 36: D1.
Havron, A., Rosen, D., Rössler, Y., & Hillel, J. (1987). Selection on the male hemizygous genotype in arrhenotokous insects and mites. Entomophaga, 32: 261-268.
James, D. G., & Price, T. S. (2002). Fecundity in two spotted spider mite (Acari: Tetranychidae) is increased by direct and systemic exposure to imidacloprid. Journal of Economic Entomology, 95: 729–732.
Jeschke P, Nauen R (2008). Neonicotinoids - from zero to hero in insecticide chemistry. Pest Manag. Sci., 64: 1084-1098.
Han, Y., Wang, Y., Bi, J. L., Yang, X. Q., Huang, Y., Zhao, X., & Cai, Q. N. (2009). Constitutive and induced activities of defense-related enzymes in aphid-resistant and aphid-susceptible cultivars of wheat. Journal of chemical ecology, 35: 176-182.
Hodgson, Erin W. (2020). Scouting reminders for spider mites. Integrated Crop Management News, 2647.
Kaplan, I. (2012). Attracting carnivorous arthropods with plant volatiles: the future of biocontrol or playing with fire?. Biological control, 60: 77-89.
Kaplan, I., & Lewis, D. (2015). What happens when crops are turned on? Simulating constitutive volatiles for tritrophic pest suppression across an agricultural landscape. Pest Management Science, 71: 139-150.
Kaplan, I., Thaler, J.S., (2010). Plant resistance attenuates the consumptive and non- consumptive impacts of predators on prey. Oikos, 119: 1105–1113.
Kessler, A., & Baldwin, I. T. (2000). Defensive function of herbivore-induced plant volatile emissions in nature. Science, 291: 2141-2144.
Law SE (2001). Agricultural electrostatic spray application: a review of significant research and development during the 20th century. J. Electrost., 51–52: 25–42.
Li, H., Payne, W. A., Michels, G. J., & Rush, C. M. (2008). Reducing plant abiotic and biotic stress: Drought and attacks of greenbugs, corn leaf aphids and virus disease in dryland sorghum. Environmental and Experimental Botany, 63: 305-316.
Maag, D., Köhler, A., Robert, C. A., Frey, M., Wolfender, J. L., Turlings, T. C., & Erb, M. (2016). Highly localized and persistent induction of Bx1‐dependent herbivore resistance factors in maize. The Plant Journal, 88:976-991.
Martin, D. E., & Latheef, M. A. (2019). Aerial application methods control spider mites on corn in Kansas, USA. Experimental and Applied Acarology, 77: 571-582.
Martini X, Kincy N, Nansen C (2012) Quantitative impact assessment of spray coverage and pest behavior on contact pesticide performance. Pest Manag. Sci., 68: 1471-1477.
Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Sattelle DB (2001). Neonicotinoids: insecticides acting on insect nicotinic acetylcoline receptors. Trends Pharmacol. Sci., 22: 573-580.
Meihls, L.N., Kaur, H., Jander, G., (2012). Natural variation in maize defense against insect herbivores, in: Cold Spring Harbor Symposia on Quantitative Biology. Cold Spring Harbor Laboratory Press, pp. 269–283.
Migeon A, Dorkeld F (2019). Spider Mites Web: A comprehensive database for the Tetranychidae. https :// www.montp ellie r.inra.fr/CBGP/spmwe b. Acessed 20 Jul, 2019.
Miotto, J., Duarte, A. F., Bernardi, D., Ribeiro, L. P., Andreazza, F., & Cunha, U. S. (2020). Toxicities of acetogenin-based biomiticides against two-spotted spider mite and selectivity to its phytoseiid predators. Experimental and Applied Acarology, 81: 173-187.
Moser SE, Obrycki JJ. (2009). Non-target effects of neonicotinoid seed treatments; mortality of coccinellid larvae related to zoophytophagy. Biol. Control, 3:487-92
Munthali DC (1984) Biological efficiency of small dicofol droplets against Tetranychus urticae (Koch) eggs, larvae and protonymphs. Crop Protect., 3: 327–334.
Nauen R, Bretschneider T, Elbert A, Fisher R, and Tieman R (2003). Spirodiclofen and spiromesifen. Pest. Outlook, 14: 243-246.
Peairs, F. (2012). Spider Mites in Corn. Fact Sheet, 5.555, 3.
Peairs, F.B., Both, B.G.M., (2010). Spider mites in corn. Colorado State University.
Pereira, J. F., Sarria, A. L., Powers, S. J., Aradottir, G. I., Caulfield, J. C., Martin, J., & Pereira, P. R. (2017). DIMBOA levels in hexaploid Brazilian wheat are not associated with antibiosis against the cereal aphids Rhopalosiphum padi and Sitobion avenae. Theoretical and Experimental Plant Physiology, 29: 61-75.
Pickett, C. H., & Gilstrap, F. E. (1986). Natural enemies associated with spider mites (Acari: Tetranychidae) infesting corn in the High Plains region of Texas. Journal of the Kansas Entomological Society, 524-536.
Pralavorio, M., Fournier, D., & Millot, P. (1989). Activité migratoire des tétranyques: mise en évidence d'un rythme. Entomophaga, 34: 129-134.
Riedl H, Johansen E, Brewer L., Barbour J (2006). How to reduce bee poisoning from pesticides. A Pacific North-West Extension Publication PNW 591.
Ruckert, A., Golec, J. R., Barnes, C. L., & Ramirez, R. A. (2021). Banks Grass Mite (Acari: Tetranychidae) Suppression May Add to the Benefit of Drought-Tolerant Corn Hybrids Exposed to Water Stress. Journal of Economic Entomology, 114: 187-196.
Ruckert, A., & Ruckert, A. (2017). DigitalCommons @ USU Interactions Between Plant Water-Stress and Neonicotinoid Insecticides on Spider Mite Infestations in Corn.
Ruckert, A., and Ramirez R.A., (2015). Combined effects of drought-stress and neonicotinoid seed treatment on Banks grass mite (Oligonychus pratensis) in corn. Utah State University Research Week, Logan, UT, 18-20 March. Ruckert,
Sabelis, M. W., & Dicke, M. (1985). Long-range dispersal and searching behaviour. January 2016.
Sáenz-de-Cabezón Irigaray, F. J., Zalom, F. G., & Thompson, P. B. (2007). Residual toxicity of miticides to Galendromus occidentalis and Phytoseiulus persimilis reproductive potential. Biological Control, 40: 153-159.
Schmidt, R. A. (2014). Leaf structures affect predatory mites (Acari: Phytoseiidae) and biological control: A review. Experimental and Applied Acarology, 62: 1-17.
Scholer J, Krischik V. (2014). Chronic exposure of imidacloprid and clothianidin reduce queen survival, foraging, and nectar storing in colonies of Bombus impatiens. PLoS One, 9: e91573.
Singh, B. U., & Seetharama, N. (2008). Host plant interactions of the corn planthopper, Peregrinus maidis Ashm.(Homoptera: Delphacidae) in maize and sorghum agroecosystems. Arthropod-Plant Interactions, 2:163-196.
Stumpf N, and Nauen R (2001). Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari Tetranychidae). J. Econ. Entomol., 94: 1577-1583.
Yu, X., Zhang, Y., Liu, Y., Li, Y., & Wang, Q. (2019). Synthesis and Acaricidal- and Insecticidal-Activity Evaluation of Novel Oxazolines Containing Sulfiliminyl Moieties and Their Derivatives [Research-article]. Journal of Agricultural and Food Chemistry, 67: 4224-4231.
Van der Sluijs JP, Simon-Delso N, Goulson D, Maxim L, Bonmatin J, Belzunces LP (2013). Neonicotinoids, bee disorders and the sustainability of pollinator services. Cur. Opin. in Environ. Sustain., 5: 293-305.