Abstract
The prevalence of parasite populations resistant to commercial broad-spectrum anthelmintic drugs has been dramatically increasing and suggests the need to adopt new prophylaxis strategies. In order to overcome this problem, validation of new anthelmintic molecules from plant extracts were performed and many in vitro and in vivo bioassays were published. In the present article, we will first review several isolation methods of different stages of parasite materials that are needed to perform in vitro bioassays. Then an overview of different techniques of in vitro and in vivo screening assays used to evaluate anthelmintic activity of the plant extracts against gastrointestinal nematodes is presented.
Keywords: Laboratory bioassay, gastrointestinal nematodes, plant extracts
INTRODUCTION
During the twentieth century, helminths affections are the cause of considerable economic losses in livestock around the world. This is by affecting negatively animals’ food intake, digestion, absorption and utilization (Jackson et al., 2009). Henceforth, different methods to control helminths in farm animals have been used. The chemical control is the most widely adopted option throughout the world as it is the cheapest and the simplest therapeutic and prophylactic cover against helminths. However, the resistance to these drugs has been dramatically increasing and suggests the need to adopt new prophylaxis investigations (Jackson and Miller, 2006). Moreover, the increased awareness of consumers about environmental and food drug residues along with limited affordability and availability to many herders in the developing countries has stimulated studies into alternative strategies of gastrointestinal parasitism control in ruminants (Hammond et al., 1997).
Examination of several plant extracts for their potential to reduce or remove gastrointestinal nematodes in livestock have been thoroughly investigated (Hrckova and Velebny, 2013). Considerable efforts have been made to identify the active ingredients so that some of them are well known nowadays (Tagboto and Townson, 2001). In order to validate anthelmintic activity of plant extracts, many in vitro and in vivo bioassays were performed. For this purpose, researchers first perform a primary screening of plants that need investigation in their area. Both in vitro and in vivo assays can be used as methods for primary screening, but the use of in vitro assays is more feasible than in vivo assays due to the time and financial cost that are involved for its implementation, especially with large scale screening of plants (Jackson and Gordon, 2008). In the present article, we will first review several isolation methods of different stages of parasite materials that are needed to perform in vitro bioassays and then an overview of different techniques of in vitro and in vivo screening assays used to evaluate anthelmintic activity of plant extracts against gastrointestinal nematodes is given.
PREPARATION METHODS OF PARASITE MATERIALS: COLLECTION OF DIFFERENT STAGES OF NEMATODES
Many procedures and techniques are used to isolate and culture different stages of nematodes parasites (eggs, L1, L3 and adult worms) in order to perform different in vitro bioassays. Fecal material can be collected from animals naturally infested with gastrointestinal nematodes or experimentally infected with specific species.
Nematode eggs recovery technique
Several methods were described by many authors in order to isolate nematode eggs with a clear preparation for the use in egg hatch tests or for culturing of first stage (L1) larvae. This consists of thoroughly dispersing fecal material in tap water before sieving. Then, saturated salt solution is used to clean parasite eggs by floatation.
Eggs of nematodes can be recovered using the technique described by Le Jambre (1976). Feces obtained from the sheep are suspended in water, and saturated NaCl solution was used for washing them through the sieve. A shallow tray with the depth of 4 cm was used to collect the mixture and a sheet of plastic cut to the shape of the tray was floated on top. Then some nematode eggs with less gravity float to the top to adhere to the plastic sheet. The eggs are recovered by washing the plastic sheet after about 15 min into a beaker. Cole et al. (1992) have used centrifugation where the suspension of the fecal material is poor through a 100 mesh and centrifuged for 2 min at 300 g. A published data paper by Dryden et al. (2005) found that the better way to recover more worm eggs is centrifugation compared to other methods like direct smears or letting samples set for long periods of time. The supernatant is gently discarded, and saturated sodium chloride solution is added to the sediment until a meniscus forms above the tube and recovered with cover slip before another centrifugation. The cover slip is washed off to recover eggs into a conical tube and then filled with water. A clear preparation can be obtained by putting the suspension under running water through a series of overlapping sieves (500, 150, 90, and 20 µm). Eggs retained on the last sieve are recovered with super saturated saline solution by simple flotation and washed three times in distilled water by use of centrifugation (Jackson and Gordon, 2008). Then, the concentration of eggs is estimated under stereo microscope by using the modified McMaster method. Larval development needs bacteria (Bouchet et al., 2022). This can be provided by adding centrifuged filtrate (5 min at 100 g) from the first step of egg extraction to the suspension. Proliferation of fungi is avoided by adding 5 μg of amphotericin B per mL of suspension (Le Jambre, 1976).
Other solutions, other than saturated salt solution, can be used to recover nematodes eggs (Table 1) (Dryden et al., 2006). A research conducted by Bliss (2007) found that recovering large number of eggs from different species is better with the Sheather’s sugar solution used with the Modified Wisconsin Sugar Flotation Technique compared to other solutions.
Some authors prefer to obtain fresh mono-species eggs for egg hatch and larval development assays by crushing gently in PBS with pestle and mortar adult nematode females harvested from the gastrointestinal contents of sheep (Al-Shaibani et al., 2009; Kakar et al., 2013). Others prefer to isolate the eggs from the adult female worms by incubating them at 37°C for one hour in normal saline and centrifugating at 2000 rpm for two minutes (Mini et al., 2013).
Culture of first stage (L1) larvae
First stage larvae can be used in two kinds of methods: larvicidal assays and the larval feeding inhibition assay. Nematode eggs recovered can be cultured in 24 well plates or in Petri dishes, which provides a better condition for the eggs to hatch by allowing large gas surface exchange. This can be done at room temperature or in an incubator for 24 h until hatched to first stage larvae (L1) (Mini et al., 2013). Baermann method is used to remove debris and unhatched eggs (Jackson and Hoste, 2010).
Culture of third stage (L3) larvae
The effect of a test substance on locomotion of infective larvae is called the larval migration inhibition assay. To obtain parasite materials for this method, the fecal samples have to be collected directly from the rectum of infested animals and incubated at 25°C for 7-10 days in Petri dish cultures. Infective larvae can be visible in the peripheral part of the culture using a standard microscope (Moskwa et al., 2014). Then, larvae are collected from pellets using Baermann technique after flooding feces by water (Jackson and Hoste, 2010). Determination of different species’ prevalence has to be considered due to the large sensitivity differences to bioactive substances between nematode species collected from field infections (Van Wyk and Mayhew, 2013).
Collection of adults worms
Adult worms are used for adulticide trials and larval migration inhibition assays. They can be isolated from gastrointestinal contents by recovering them manually from sheep abomasa after 10 to 20 weeks post-infection. They are then placed in culture medium containing RPMI-1640, Hepes buffer, glucose, bovine serum, antibiotics, and fungicide for several hours before the running of the test (Kotze and McClure, 2001). 20% of newborn bovine serum can be included in the culture medium (O’Grady and Kotze, 2004). Adult worms can be collected also by soaking the mixture of agar-water with the gastrointestinal contents. They can be recovered from water after their migration from the agar slab (Jackson and Hoste, 2010).
DIFFERENT BIOASSAYS FOR THE ASSESSMENT OF PLANT EXTRACTS’ ANTHELMINTIC ACTIVITY
In vitro screening assays
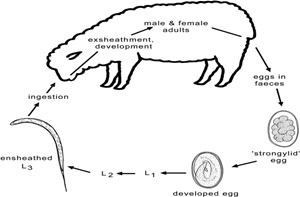
Several in vitro studies were performed in order to evaluate the anthelmintic activity of plant extracts (Figure 1), confirming its scientific validation and supporting the hypothesis of direct anthelmintic effects of plant products (Brunet and Hoste, 2006). In general, in vitro screening techniques were developed from those used to measure the effectiveness of the chemical drugs (Coles et al., 1992) and are performed to evaluate the mortality, motility and feeding behavior of the parasites after treatment with plant extracts. Besides their low costs and rapid turnover, in vitro bioassays have the advantage to allow large scale screening of plants and their purified compounds to avoid interference with other plant components (Athanasiadou and Kyriazakis, 2004).
The egg hatch assay
The principle of this test is to determinate the proportion of unhatched eggs following their contacts with the increasing concentration of the tested product in relation to the control wells. Then, a dose response line can be plotted against the concentration of tested extracts. This in turn allows the determination of the ED50 which is the concentration of product that is required to inhibit 50% of the eggs from hatching (FAO, 2004). Benzimidazole analogues of intermediate polarity have been reported to induce ovicidal effects. Indeed, the more polar and more hydrophobic analogues are, the less penetration of the eggshell is observed (Lacey, 1988). On account of the emergence of the resistance against this chemical drug, it is important to explore other alternative molecules. The test is usually carried out on nematode species eggs that have the characteristic to hatch rapidly and following the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines (Coles et al., 1992) with some modifications for testing plant extracts (Alawa et al., 2003).
Briefly, 100 µl of the suspension of freshly extracted eggs is distributed in a 24-well flat-bottomed microtitre plate and mixed with the same volume of different concentrations of the tested plant extracts. A solvent control that was used for preparing plant extract and a positive control (thiabendazole) have to be included in the assay. Then, incubation for 48 hours is done at room temperature and the eggs have to be stopped from hatching by adding a drop of Lugol’s iodine solution. The numbers of hatched larvae and unhatched eggs are counted in each well using an inverted or stereo microscope (Jackson and Gordon, 2008).
Larval migration inhibition test (LMI)
This assay was first developed by Wagland et al. (1992) then modified by Rabel et al. (1994) and used to evaluate the inhibition of third stage larvae nematodes passage through 20 µm nylon mesh sieves by paralyzing them using a product (plant extracts).This process may have similarities to L3 penetration of the gut mucus to reach the mucosa (Rabel et al., 1994). This inhibition would only result from effects on the musculature of the body wall’s larvae as they don’t feed (Gill et al., 1995).
A suspension of third stage larvae is added to 15 ml centrifuge tubes and a range of concentrations of the tested substance of each solution to be tested is added and incubated for 3 h at 21.3 °C. The test must contain a negative control (PBS; pH 7.2) and positive control data can be provided using the anthelmintic levamisole. PBS is then added to the tested tubes and centrifugated three times at 2500 rpm to wash Larvae. The preparation is then sieved using inserts equipped with a 20 µm mesh positioned in a conical tube and incubated for 3 h at room temperature. Following this, a counting of migrated L3 through the mesh can be performed. The percentage of migration is calculated as M/T *100 where T represent the total number of L3 deposited on the sieve and M the number of migrated L3 (Von Son-de Fernex et al., 2012; Kotze et al., 2006). Some authors advise to use multiwell plates and an inverted stereo microscope as a saving time method to run the test (Jackson and Gordon, 2008).
Larval exsheathment assay (LEA)
The trichostrongyle nematodes have a specific keyprocess in the life cycle which is the L3 exsheathment. This means transition from the free-living to the parasitic stages, which is a rapid critical step that has to occur without any disturbing factors (Kotze et al., 2006). This is why it is important to perform tests which use infective L3 in both processes to test the effect of the plant extract on larval exsheathment. The aim is to assess the kinetics of exsheathment of the third stage larvae using microscopic observation of the process. A 100% exsheathment after 60 to 70 minutes is considered as a positive control while PBS and extracts of non-bioactive forage can be considered as negative controls (Jackson and Gordon, 2008).
Briefly, a large number of ensheathed L3 are incubated with the tested plant material at a concentration of 1200 µg/ml PBS for 3 h at 21 °C. Then, the larvae are washed in PBS (pH 7.2) by centrifugation three times (2500 rpm). Following this, an artificial exsheathment process is induced using a solution of sodium hypochloride (2%) diluted 1:300 in PBS (pH 7.2) (Alonso-Díaz et al., 2008). Exsheathed larvae are counted at different times in each experimental treatment (0, 10, 20, 30, 40, 50 and 60 min) by microscopic observation (40x)(Von Son-de Fernex et al., 2012).
Larval feeding inhibition test (LFI)
This assay was firstly described by Jackson and Coop (2000) and Álvarez-Sánchez et al. (2005) and consists on evaluating the reduction of food ingestion by first stage larvae (L1) by exposing them to serial dilutions of the tested plant extract for 2 to 4 hours at 24°C. Lyophilized Escherichia coli labeled with florescent compound (fluorescein 5-isothiocyanate or FITC) are added as nutritive medium before incubation at 24°C for 24 hours. The percentage of larvae fed for each dilution is estimated using an inverted fluorescence microscope by examining fluorescence from the labeled E. coli coming from the larvae’s intestine. The Dose of Larval Feeding Inhibition 50 -DLFI50- which means the concentration of the tested product needed to inhibit the ingestion in 50% of the L1, is further calculated (Álvarez-Sánchez et al., 2002). Controls and either ivermectin or levamisole positive control must be included in the assay.
Larval development inhibition assay (LDI)
This assay evaluates the ability of the parasite to hatch and develop to the infective third stage larvae in the presence of the tested product by using fresh eggs or L1 (Jackson and Gordon, 2008). In this assay, the tested product may be ingested by the larvae as they are in the feeding stage of their life cycle (Molan et al., 2003). Antibiotics and antifungals must be incorporated due to the long period of incubation involved in this assay.
A solution of nematodes eggs is put into each well of 96-well microtitre plate with nutritive media and incubated for 2 days at room temperature. Following this, plant extracts in different concentrations are added to relevant plates then further incubated for 5 days. One drop of Lugol's iodine solution is added to stop larvae’s development before counting them under stereo microscope (Suteky and Dwatmadji, 2011).
Adult motility assay (AMA)
Freshly recovered female mature live worms as described previously are suspended in PBS or a culture medium, and then placed into tubes where tested plant products at different concentration are added. The tubes are incubated at 37 °C with 5% CO2 in air (O’Grady and Kotze, 2004).
The number of active worms is monitored and calculated at regular times during the assay (at 0 hour and then after 6, 24, 48 and 72 hour intervals). To evaluate the motility recovery, immobile worms have to be kept for 30 minutes in the lukewarm fresh PBS until stimulation of sinusoidal motion movements. Positive control should be incorporated like Oxfendazole or ivermectin and distilled water is used as negative control (Jackson and Hoste, 2010).
In vivo screening assays
Animals used can be experimentally infected with a known amount of specific parasite species or naturally infected carrying several species. In the first case, animals have to spend a period of adaptation to the housing conditions and treated with a chemical anthelmintic in order to ensure helminth-free conditions and then, after one month, a known number of third stage larvae of specific parasite species are inoculated orally (Hördegen et al., 2003). In the last case, coproculture with larval determination or PCR based speciation techniques should be performed in order to highlight a possible specific effect of the plant extract tested (Jackson and Gordon, 2008).
In vivo studies of the anthelmintic effect of plant extracts can be done using two methods. The indoor supplementation method investigates animals who are receiving controlled amounts of the tested material (Akhtar and Ahmad, 1992). These can be administered orally using a syringe or as powder mixed with aliment (barley grain…). The second method consists on putting animals to graze on forage rich in tested plant which simulate normal agricultural practice (Marley et al., 2003).
These studies should be performed with a sufficient number of animals that can provide power to statistical calculations in fecal egg count reduction. The WAAVP guidelines for testing anthelmintic drug efficacy is used by studies evaluating plant anthelmintic product (Wood et al., 1995) and recommend the presence of at least six animals per group (Vercruysse et al., 2001).
Various in vivo assays are usually performed in order to evaluate the activity of plant extracts in animals. These include fecal egg count or daily fecal egg excretion after treatment with the tested plant extract using modified McMaster-technique seven days after treatment (Hördegen et al., 2003). Expulsion of worms and counting of parasites in feces (Akhtar and Riffat, 1986) as well as regular worm counts in necropsied animals (Akhtar and Ahmad, 1992) were reported too. Besides, it is necessary to include an untreated group to monitor natural changes in egg counts during the test, which may sometimes not be practical in field conditions. Also, a group treated with the carrier or the solvent used with the tested extract should be incorporated in the study (Jackson and Gordon, 2008).
CONCLUSION
A large-scale plant screening assay should be performed in order to discover new anthelmintic molecules (O’Grady and Kotze, 2004). In vitro studies had provided much scientific evidence that support the antiparasitic properties of plant extracts. However, the majority of these assays have been mainly developed to detect resistance of nematodes to the currently used anthelmintics and also to evaluate the efficacy of new synthesized chemical anthelmintics. These bioassays offer many advantages such as the possibility to test purified compounds, and the rapid turnover with low cost that allows large-scale plant screening. However, in vivo studies are considered as the most relevant investigations than in vitro studies since they show the normal agricultural situation in which plant extracts can exert their effects (Athanasiadou and Kvriazakis, 2004).
The results of assessing anthelmintic activity of plants can differ in the two assays because of many reasons. Concentrations of the tested materials are not the same used in in vitro and in vivo assays. Moreover, there are some physiological differences that must be considered between in vitro conditions, the predilection site of the parasite within their animal hosts and internal factors that affect bioavailability of the tested products at different parts of the gastrointestinal tract (Githiori et al., 2006). In in vitro studies, the parasite material is in direct contact with the tested extract. Whereas, in in vivo studies some habitat niche of certain stages of some parasites, like Teladorsagia circumcincta, which is an abomasal nematode of sheep that parasites on the gland of abomasum, may reduce the contact with the plant extracts and could make them appear inactive in vivo (Urquart et al., 1996). Furthermore, certain plant extracts that showed in vitro activity could be inactive in vivo as a result of the gastrointestinal tract conditions. Thus they can show selective interspecies difference regarding activity (Athanasiadou et al., 2005). For instance, condensed tannin shows strong antiparasitic activity against abomasal nematodes of sheep in vitro, but no effect against them in vivo. This can be explained by the fact that condensed tannins are most of the time in complexes with proteins due to their high affinity to them. The complexes are expected to break down in the small intestine so that the tannins will be active against intestinal parasites (Athanasiadou and Kvriazakis, 2004).
In conclusion, in vitro tests have many advantages providing an interesting means for large-scale plant screening, whereas in vivo screening is not as effective due to time and financial constraints involved. It is, however, mandatory to validate both in vitro and in vivo results before making valuable conclusions on the anthelmintic activities of the plant extracts assessed.
REFERENCES
Akhtar M.S. and Ahmad I. (1992). Comparative efficacy of Mallotus philippinensis fruit (Kamala) or Nilzan drug against gastrointestinal cestodes in Beetal goats. Small Ruminants Research, 8: 121–128.
Akhtar M.S., Riffat S. (1986). A field trial of Peganum harmala Linn. seeds (harmal) against natural cestodal infection in beetal goats. J. Pharmacol. (Univ. Karachi), 4: 79–84.
Alonso-Diaz M.A., Torres-Acosta J.F.J., Sandoval-Castro C.A., Hoste H., Aguilar-Caballero A.J., Capetillo-Leal C.M.(2008). Is goats’ preference of forage trees affected by their tannin or fiber content when offered in cafeteria experiments? Animal Feed Science and Technology, 141: 36-48.
Al-Shaibani, I. R. M., Phulan, M. S., Shiekh, M. (2009). Anthelmintic activity of Fumaria parviflora (Fumariaceae) against gastrointestinal nematodes of sheep. Int. J. Agric. Biol., 11: 431-436.
Álvarez-Sánchez M. A., Mainar Jaime R. C., Perez Garcia J., Rojo Vazquez F. A. (2002). A review of the methods for the detection of anthelmintic resistance. Research and Reviews in Parasitology, 62: 51-59.
Alvarez-Sánchez M. A., Pérez García J., Bartley D., Jackson F., Rojo-Vázquez F. A. (2005). The larval feeding inhibition assay for the diagnosis of nematode anthelmintic resistance. Experimental Parasitology, 110: 56–61.
Athanasiadou S., Tzamaloukas O., Kyriazakis I., Jackson F., Coop R.L. (2005). Testing for direct anthelmintic effects of bioactive forages against Trichostrongylus colubriformis in grazing sheep. Veterinary Parasitology, 127: 233–243.
Athanasiadou S., Kvriazakis I. (2004). Plant secondary metabolites: antiparasitic effects and their role in ruminant production systems. Proceedings of the Nutrition Society, 63:631-639.
Bliss D.H. (2007). The control of gastro-intestinal nematode parasites in horses with emphasis on reducing environmental contamination: A new control strategy for an old problem. Mid America Agricultural Research: 1-23.
Bouchet C., Deng Q., Umair S. (2022). Bacteria Associated with the Parasitic Nematode Haemonchus contortus and Its Control Using Antibiotics. Parasitologia, 2: 63-70.
Bożena M., Justyna B., Katarzyna G., Władysław C. (2014). The usefulness of DNA derived from third stage larvae in the detection of Ashworthiussidemi infection in European bison by a simple polymerase chain reaction. Parasites & Vectors, 7: 215.
Brunet S., Hoste H. (2006). Monomers of condensed tannins affect the larval exsheathment of parasitic nematodes of ruminants. J. Agric. Food Chem., 54: 7481-7487.
Coles G.C., Bauer C., Borgsteede F.H.M., Geerts S., Klei T.R., Taylor M.A., Waller P.J. (1992). World Association for the Advancement of Veterinary Parasitology methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol., 44 : 35–44.
Dryden M.W., Payne P.A., Ridley R. et al. (2005). Comparison of common fecal flotation techniques for the recovery of parasite eggs and oocysts. Vet Ther., 6:15-28.
FAO (2004). Module 2. Helminths: Anthelmintic Resistance: Diagnosis, Management and Prevention. Guidelines Resistance Management and Integrated Parasite Control in Ruminants. Food and Agriculture Organization of the United Nations, Rome.
Gill J.H., Redwin J.M., Van Wyk J.A., Lacey E. (1995). Avermectin inhibition of larval development in Haemonchuscontortus: Effects of ivermectin resistance. Int. J. Parasitol., 25:463-470.
Githiori J.B., Athanasiadou S., Thamsborg S.M.(2006). Use of Plants in Novel Approaches for Control of Gas-trointestinal Helminths in Livestock with Emphasis on Small Ruminants. Veterinary Parasitology, 139: 308-320.
Hammond J.A., Fielding D., Bishop S.C. (1997). Prospects for plant anthelmintics in tropical veterinary medicine. Veterinary Research Communications, 21: 213-228.
Hördegen P., Hertzberg H., Heilmann J., Langhans W., Maurer V. (2003). The anthelmintic efficacy of five plant products against gastrointestinal trichostrongylids in artificially infected lambs. Vet. Parasitol., 117: 51–60.
Hrckova G., Velebny S. (2013). Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases. Springer Briefs in Pharmaceutical Science & Drug Development.
Jackson F., Bartley D., Bartley Y., Kenyon F. (2009). Worm control in sheep in the future. Small Ruminant Research, 86: 40-45.
Jackson F., Coop R.L. (2000). The development of anthelmintic resistance in sheep nematodes. Parasitology, 120: 95-107.
Jackson F., Miller J. (2006). Alternative approaches to control-Quo vadit? Vet. Parasitol., 139: 371–384.
Jackson F., Gordon Y. (2008). Screening plants for anthelmintic activity: A challenging situation. In: Congressobrasileiro de parasitologia veterinária, 25.; Seminário de parasitologia veterinária dos países do mercosul, 2. 2008, Curitiba. Programa E Resumos. Curitiba: Ufpr: Universidad e Estadual De Londrina. 11 F. 1 Cd-Rom.
Jackson F., Hoste H.(2010). In vitro methods for primary screening of plant products direct activity against ruminant gastrointestinal nematodes. In: Vercoe P. E., Makkar H. P. S., Schlink A. C. In vitro screening of plants resources for extra-nutritional attributes in ruminants: nuclear and related methodologies. London: Springer: 25-45.
Kakar S.A., Tareen R.B., Sandhu Z.U., Azam M.K., Saeed U.K., Zafar I., Jabeen H. (2013). In vitro and in vivo anthelmintic activity of Ferula costata (Kor.) against gastrointestinal nematodes of sheep, Pak. J. Bot., 45: 263-268.
Kotze A.C., Le Jambre L.F., O Grady J. (2006). A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol., 137: 294-305.
Kotze A.C., McClure S.J.(2001). Haemonchus contortus utilises catalase in defence against exogenous hydrogen peroxide in vitro. International Journal for Parasitology, 31: 1563–1571.
Lacey E. (1988). The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. International Journal for Parasitology, 18: 885-936.
Le Jambre L.F. (1976). Egg hatch as an in vitro assay of thiabendazole resistance in nematodes. Vet. Parasitol., 2: 385–391.
Marley C.L., Cook R., Keatinge R., Barrett J., Lampkin N.H. (2003). The effect of birdsfoot trefoil (Lotus corniculatus) and chicory (Cichorium intybus) on parasite intensities and performance of lambs naturally infected with helminth parasites. Veterinary Parasitology, 112: 147–155.
Mini K.P., Venkateswaran K.V., Gomathinayagam S., Selvasubramanian S., Bijargi S.R. (2013). In vitro Anthelmintic Effect of Aqueous and Ethanol Extract of Aristolochia indica Against Haemonchus contortus. J. Phys. Pharm. Adv., 3: 148-158.
Molan A.L., Meagher L.P., Spencer P.A., Sivakumaran S. (2003). Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Int. J. Parasitol., 33:1691–1698.
O’Grady J., Kotze A.C. (2004). Haemonchus contortus: in vitro drug screening assays with the adult life stage. Experim. Parasitol., 106: 164-172.
Rabel B., McGregor R., Douch P.G. (1994). Improved bioassay for estimation of inhibitory effects of ovine gastrointestinal mucus and anthelmintics on nematode larval migration. Int. J. Parasitol., 24:671-676.
Tagboto S., Townson S. (2001). Antiparasitic properties of medicinal plants and other naturally occurring products. Adv Parasitol., 50:199-295.
Suteky T., Dwatmadji T. (2011). Anthelmintic activity of Melastoma malabatricum extract on Haemonchus contortus activity in vitro. Asian J. Pharm. Clin. Res., 4: 68-70.
Urquart G.M., Armour J., Duncan J.L., Dunn A.M., Jennings F.W.(1996). Veterinary Helminthology. In Parasitology: 10–26 [GM Urquart, editor]. Oxford: Blackwell.
Van Wyk J.A., Mayhew E. (2013). Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort Journal of Veterinary Research, 80: 1-14.
Vercruysse J., Holdsworth P., Letonja T., Barth D., Conder G., Hamamoto K., Okano K.(2001). International harmonization of anthelmintic efficacy guidelines. Vet. Parasitol., 96: 171– 193.
Von Son-deFernex E., Alonso-Díaz M.A., Valles-de la Mora B., Capetillo-Leal C.M. (2012). In vitro anthelmintic activity of five tropical legumes on the exsheathment and motility of Haemonchus contortus infective larvae. Exp. Parasitol., 131:413–418.
Wagland B.M., Jones W.O., Hribar L., Bendixsen T., Emery D.L. (1992). A new simplified assay for larval migration inhibition. Int. J. Parasitol., 22:1183-1185.
Wood I.B., Amaral N.K., Bairden K., Duncan J.L., Kassai T., Malone Jr. J.B., Pankavich J.A., Reinecke R.K., Slocombe O., Taylor S.M. (1995). World Association for the Advancement of Veterinary Parasitology second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine). Vet. Parasitol., 58: 181–213.